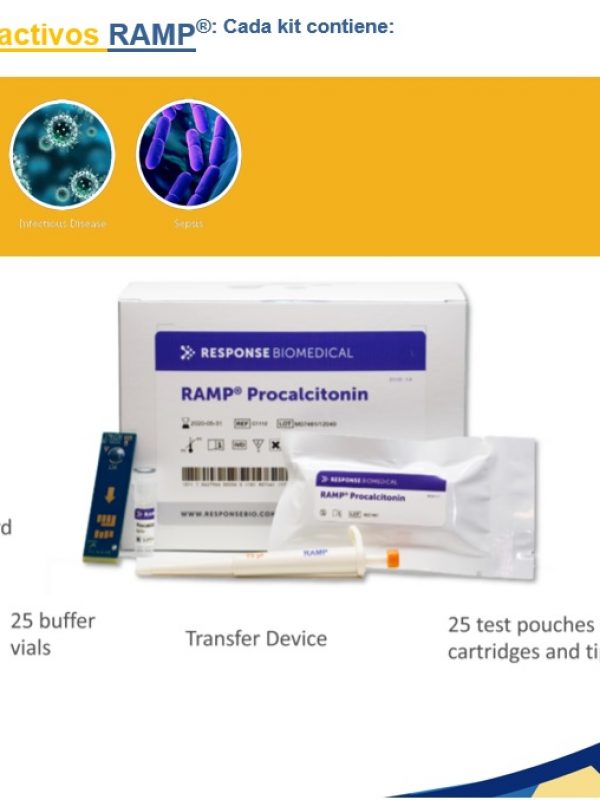
- Language configuration (Spanish, English, Portuguese, French, Italian).
- Language configuration (Spanish, English, Portuguese, French, Italian).
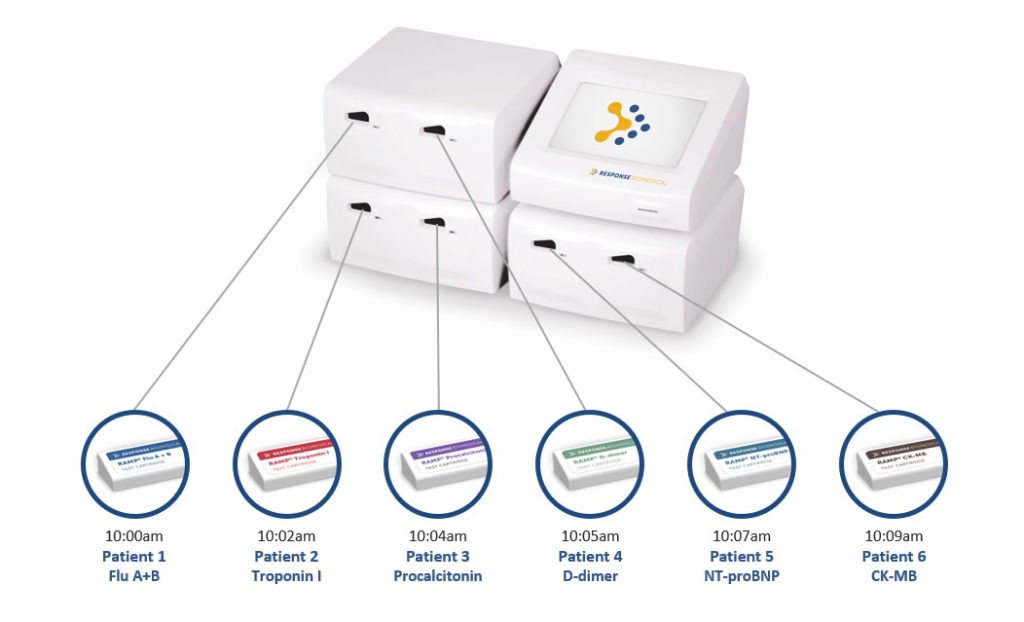
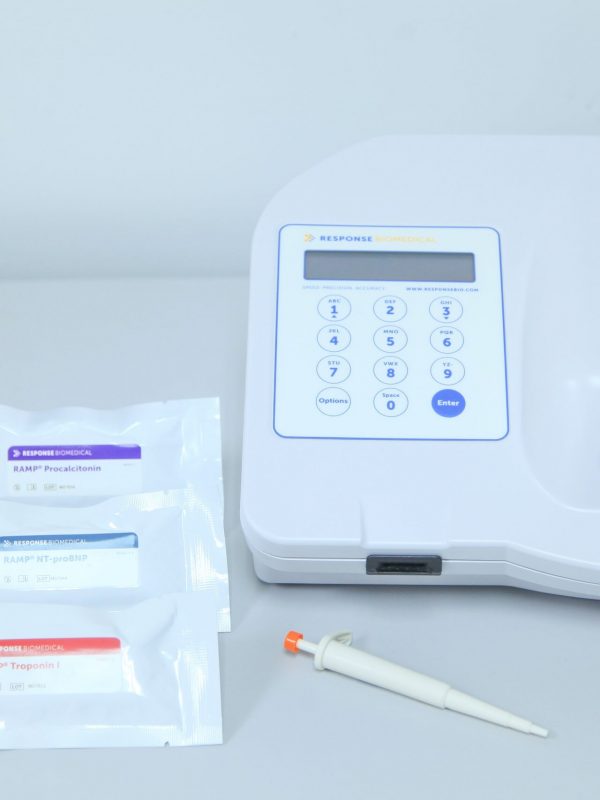
- Designed for a high volume of work (up to 36 tests per hour).
- Memory for up to 900 tests with counter reset option. Hematocrit (Hct) reading
- Capable of performing multiple tests on different patients at the same time.
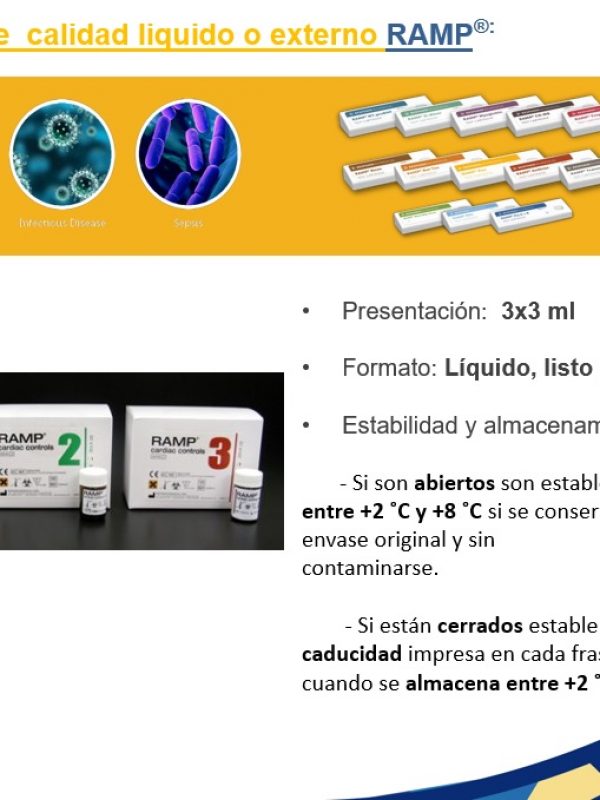
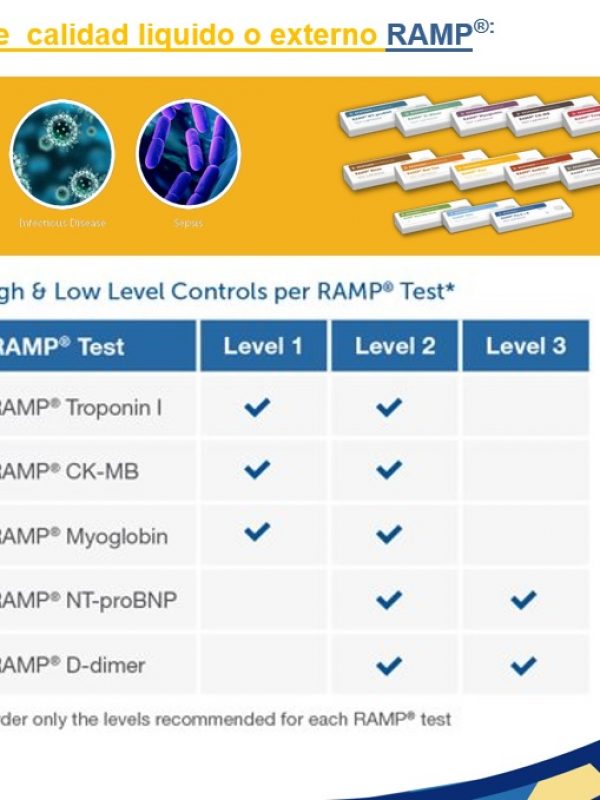
- Integrated internal quality controls.
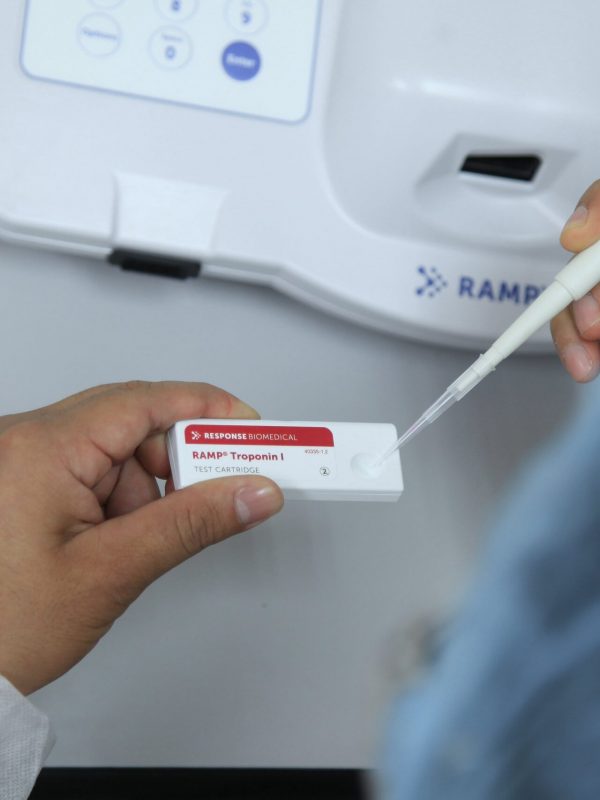
- It does not require calibration and maintenance.
- Multiple levels of access according to user permissions to offer greater security.